Purification project
热门关键字:食品净化车间 医药洁净车间 低温车间 洁净室 无尘洁净室
Color cosmetics cleanroom-Meliorite
Category:
Project Description
MetriCleanroomRenovation work
Project Name:Ningbo Metri Cosmetic Co.
Project content:100,000 class purification project
Industry Category:Cosmetic purification project

■About Metri
Ningbo Metri Cosmetic Co. office address is conveniently located in No. 888, Fengnan Road, Jinping Street, Fenghua City, Zhejiang Province, was registered in Ningbo Fenghua District Market Supervision Administration on August 29, 2008, in the company's 11 years of development and growth, we always provide customers with good products and technical support, sound after-sales service, our company mainly engaged in Beauty and decoration cosmetics production (in ... Manufacture and processing of cosmetic plastic boxes, special equipment for daily use chemicals, cutting and processing of organic glass products, wholesale of cosmetic raw materials (except dangerous chemicals and monitoring chemicals), raw and auxiliary materials for machinery and equipment, cosmetics, skin care products, beauty accessories, glass products, cosmetic packaging products, display cabinets; self-supporting and acting as an agent for the import and export of all kinds of goods and technologies, except for the import and export of Except for goods and technologies that are restricted or prohibited by the state.


■ Key Notes:
1、People flow and logistics
1-1、Personnel purification flow chart:
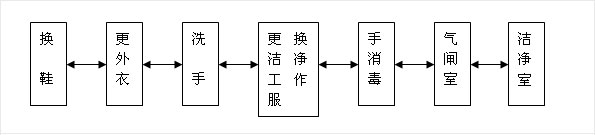
1-2, the production process should be connected up and down, human flow, logistics separate, to avoid cross. Raw materials and packaging materials, products and personnel flow routes should be clearly delineated.
2, the production line of each production plant operations, filling, packaging room total area of not less than 100 square meters; purely sub-assembly of the production plant filling, packaging room total area of not less than 80 square meters.
3, the production of eye skin care category, baby and children with skin care cosmetics semi-finished products storage room, filling room, cleaning container storage room should reach 300,000 clean requirements; other skin care cosmetics semi-finished products storage room, filling room, cleaning container storage room should reach 300,000 clean requirements.
4、Clean room air cleanliness level table
| Air cleanliness class (N) | Greater than or equal to the maximum concentration limit of the required particle size (pc/m³) | |||||
| 0.1μm | 0.2μm | 0.3μm | 0.5μm | 1μm | 5μm | |
| 1 | 10 | 2 | - | -- | -- | -- |
| 2 | 100 | 24 | 10 | 4 | -- | -- |
| 3 | 1000 | 237 | 102 | 35 | 8 | -- |
| 4 | 100000 | 2370 | 1020 | 352 | 83 | -- |
| 5 | 100000 | 23700 | 10200 | 3520 | 832 | 29 |
| 6 | 1000000 | 237000 | 102000 | 35200 | 8320 | 293 |
| 7 | -- | -- | -- | 352000 | 83200 | 29300 |
| 8 | -- | -- | -- | 3520000 | 8320000 | 29300 |
| 9 | -- | -- | -- | 35200000 | 8320000 | 293000 |
Note: According to different measurement methods, the effective digits of the concentration data of each level should not exceed 3 digits
Choose us Five advantages

Focus on food and drug, electronic industry clean room one-stop solution 18years since the establishment of a professional safety management system
18 years industry experience
1、18 years industry experience
◆ 18 years of precipitation
◆ 200 successful cases
◆ Repeat customers and word-of-mouth customers account for a high percentage
◆ Increasing number of well-known corporate clients


Construction Level 2 Qualified Enterprise
2、Construction Level 2 Qualified Enterprise
◆ Decoration Level 2 Qualification, Mechanical and Electrical Level 3 Qualification, General Contractor of Construction Level 3 Qualification
◆ Have a safety production license
◆ Self-built professional safety management system
◆ Zero major safety accidents since 18 years
3、60 technicians of all kinds
60 technicians of all kinds
◆ We can design and build high level GMP workshop class A and class 1 clean room
◆ Tailor-made design solutions
◆ Project design are strictly implement GB50073-2013\GB 50473-2008\GB50457- 2008 and other national standard clean specification and the United States federal 209E standard


Third party acceptance test verification
4、Third party acceptance test verification
◆ Self-provided full set of testing instruments, first self-test after third-party testing
◆ The project construction strictly implements national GMP, US FDA and EU Euro GMP and other domestic international standards.
Customer Focused
5、Customer Focused
◆ Complete standardized process to ensure the construction period and quality
◆ One year after the warranty period, take the initiative to visit 2 times and 1 time at home
◆ We are convinced that our work is only valuable if the users are satisfied.




