Purification project
热门关键字:食品净化车间 医药洁净车间 低温车间 洁净室 无尘洁净室
Proprietary Chinese Medicine Preparation-Renhe
Category:
Project Description
Renhe PharmaceuticalGMP Clean RoomDecoration
Project Name:Shandong Renhe Pharmaceutical Co.
Project content:Purification project
Industry Category:Pharmaceutical purification project

■Introduction of Renhe Pharmaceutical
Shandong Renhe Pharmaceutical Co., Ltd. is a state-owned pharmaceutical enterprise established in June 2001. The company is located in the national famous “Ginkgo hometown”--Tancheng territory, east of Beijing-Shanghai highway, is a comprehensive pharmaceutical enterprise with Chinese patent medicine preparation, western medicine preparation, Chinese medicine raw material extraction and health care products as the leading products. The factory covers an area of 44,000 square meters, including a construction area of 12,000 square meters. There are five production workshops for Chinese and Western medicine preparation, GBE (Ginkgo biloba extract), baicalin and other raw materials extraction of Chinese medicine, and health food. It is also equipped with constant temperature, refrigeration, quick-freezing, storage and other facilities.







■ Design basis
Quality Management Standards for Pharmaceutical Manufacturing (2010 Revision)
Drug production quality management standard (revised in 2010) (Ministry of Health Order No. 79) Appendix I
■ Key Notes:
1、Personnel purification
1-1, personnel purification: Biomedical clean room (area) of personnel purification procedures should be arranged according to the following chart
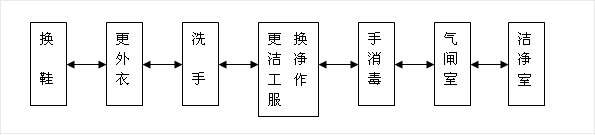
1-2, the entrance of the pharmaceutical clean area should be set up airlock room; airlock room access door should be set up interlock device.
1-3, air cleanliness level of the same sterile clean room and non-sterile clean its personnel purification with room should be set up separately.
2、Material purification
2-1, pharmaceutical clean room of raw and auxiliary materials, packaging materials entrance and exit, should be set up with material purification room.
2-2、In addition to meeting the above requirements, the raw and auxiliary materials and packages entering the aseptic clean room should also be set up at the entrance and exit of the material sterilization room and sterilization facilities.
2-3, material cleaning room or sterilization room and pharmaceutical clean room, should be set up between the airlock room or transfer cabinet.
3, 100,000 level and above area work clothes should be washed, dried and overall in the clean room, and sterilized when necessary as required.
3、Pharmaceutical clean room air cleanliness level
Pharmaceutical clean room (area) air cleanliness level:
| Air Cleanliness Grade |
Maximum allowable number of suspended particles (pcs/㎥) | Maximum allowable number of suspended particles (pcs/㎥) | ||
| ≥0.5um | ≥5um | Planktonic bacteria (cfu/ ㎥) | Settling bacteria (cfu/dish) | |
| 100 | 3500 | 0 | 5 | 1 |
| 10000 | 350000 | 2000 | 100 | 3 |
| 100000 | 3500000 | 20000 | 500 | 10 |
| 300000 | 10500000 | 60000 | — | 15 |
Choose us Five advantages

Focus on food and drug, electronic industry clean room one-stop solution 18years since the establishment of a professional safety management system
18 years industry experience
1、18 years industry experience
◆ 18 years of precipitation
◆ 200 successful cases
◆ Repeat customers and word-of-mouth customers account for a high percentage
◆ Increasing number of well-known corporate clients


Construction Level 2 Qualified Enterprise
2、Construction Level 2 Qualified Enterprise
◆ Decoration Level 2 Qualification, Mechanical and Electrical Level 3 Qualification, General Contractor of Construction Level 3 Qualification
◆ Have a safety production license
◆ Self-built professional safety management system
◆ Zero major safety accidents since 18 years
3、60 technicians of all kinds
60 technicians of all kinds
◆ We can design and build high level GMP workshop class A and class 1 clean room
◆ Tailor-made design solutions
◆ Project design are strictly implement GB50073-2013\GB 50473-2008\GB50457- 2008 and other national standard clean specification and the United States federal 209E standard


Third party acceptance test verification
4、Third party acceptance test verification
◆ Self-provided full set of testing instruments, first self-test after third-party testing
◆ The project construction strictly implements national GMP, US FDA and EU Euro GMP and other domestic international standards.
Customer Focused
5、Customer Focused
◆ Complete standardized process to ensure the construction period and quality
◆ One year after the warranty period, take the initiative to visit 2 times and 1 time at home
◆ We are convinced that our work is only valuable if the users are satisfied.




