Purification project
热门关键字:食品净化车间 医药洁净车间 低温车间 洁净室 无尘洁净室
Chinese Herbal Tablets - Tongji Tang
Category:
Project Description
Tongjitang PharmaceuticalClean room decoration project
Project Name:Shanghai Tongji Tang Pharmaceutical
Project content:Chinese medicine tablet purification project
Industry Category:Pharmaceutical purification project

■Introduction of Tongjitang
Shanghai Tongji Tang Pharmaceutical Co., Ltd. was incorporated in August 2002 and is located in Zone B of Qingpu Industrial Park, Shanghai. It is a company holding limited company mainly engaged in the production and operation of Chinese medicine tablets.The company's aim is to produce high quality Chinese medicine tablets to create health for human beings, and aspire to develop into one of the most influential Chinese medicine tablet enterprises in China. The company has strict GMP production line and quality management system, and has a standard GAP planting base in accordance with GMP.8 pharmacists who have been engaged in the processing and preparation of Chinese medicine tablets and pharmaceuticals for more than 15 years, Strong technical business force.
The purification project of traditional Chinese medicine tablets built by Shanghai Lixing reaches 10,000 levels, constant temperature and humidity control, and successfully passed the third-party authoritative testing, which meets the requirements of GMP certification.




■ Design basis
Quality Management Standards for Pharmaceutical Manufacturing (2010 Revision)
Drug production quality management standard (revised in 2010) (Ministry of Health Order No. 79) Appendix I
■ Key Notes:
1.Personnel purification
1-1, personnel purification: Biomedical clean room (area) of personnel purification procedures should be arranged according to the following chart
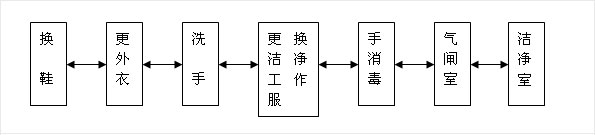
1-2, the entrance of the pharmaceutical clean area should be set up airlock room; airlock room access door should be set up interlock device.
1-3, air cleanliness level of the same sterile clean room and non-sterile clean its personnel purification with room should be set up separately.
2、Material purification
2-1, pharmaceutical clean room of raw and auxiliary materials, packaging materials entrance and exit, should be set up with material purification room.
2-2、In addition to meeting the above requirements, the raw and auxiliary materials and packages entering the aseptic clean room should also be set up at the entrance and exit of the material sterilization room and sterilization facilities.
2-3, material cleaning room or sterilization room and pharmaceutical clean room, should be set up between the airlock room or transfer cabinet.
3, 100,000 level and above area work clothes should be washed, dried and overall in the clean room, and sterilized when necessary as required.
3、Pharmaceutical clean room air cleanliness level
Pharmaceutical clean room (area) air cleanliness level:
|
Air Cleanliness |
Maximum allowable number of suspended particles (pcs/㎥) |
Maximum allowable number of microorganisms |
||
|
≥0.5um |
≥5um |
Planktonic bacteria (cfu/㎥) |
Settling bacteria (cfu/dish) |
|
|
100 |
3500 |
0 |
5 |
1 |
|
10000 |
350000 |
2000 |
100 |
3 |
|
100000 |
3500000 |
20000 |
500 |
10 |
|
300000 |
10500000 |
60000 |
— |
15 |
■ Clean room also to “ dedicated ” management:
The clean room also need “ special person ” management, regular maintenance of clean room, and not just call someone management on the line, is the need for regular maintenance management of special people. The so-called special people, is the clean room expertise, clean room maintenance professional. If the cleanroom maintenance staff is not clear and understand the basic elements of all aspects of the cleanroom, they may make some blind mistakes, clean do a good job, clean room spare parts also changed, but the cleanliness level of the cleanroom still does not meet the requirements. Therefore, cleanroom system operation and maintenance must be carried out by trained professionals.
The clean room should also be “dedicated ” management, professional clean room management should do:
1、According to the clean class of the clean room and the production process of enterprise products, develop the inspection and clean schedule of the clean room, and firmly implement.
2, inspection to the clean room of all kinds of indicators value fluctuations should be made accordingly, and immediately reflect to the person responsible for the duty.
3、Check and guide the clean room workers regularly in accordance with the clean room management system for clean, maintenance of clean processing equipment.
4、Check the operation of clean room purified air conditioning air supply system, and deal with the problems properly. When the air supply pressure is reduced to replace the primary and intermediate filters; when the air volume in the clean room drops significantly, the high efficiency filter at the end should be replaced. And develop the replacement schedule of filter consumables, and firmly implement.
5, clean room managers should contact with the project builder if they encounter a situation they cannot solve, and make emergency treatment, and so on the arrival of clean engineering technology professional enterprises.
At the end, it should be repeated that the cleanroom management should be managed by professional cleanroom maintenance managers who are trained to maintain the cleanliness level of the cleanroom to meet the needs of production at all times and to achieve high product and low residual rate is difficult. Cleanroom management by professional technicians can save a lot of material and financial resources.
Choose us Five advantages

Focus on food and drug, electronic industry clean room one-stop solution 18years since the establishment of a professional safety management system
18 years industry experience
1、18 years industry experience
◆ 18 years of precipitation
◆ 200 successful cases
◆ Repeat customers and word-of-mouth customers account for a high percentage
◆ Increasing number of well-known corporate clients


Construction Level 2 Qualified Enterprise
2、Construction Level 2 Qualified Enterprise
◆ Decoration Level 2 Qualification, Mechanical and Electrical Level 3 Qualification, General Contractor of Construction Level 3 Qualification
◆ Have a safety production license
◆ Self-built professional safety management system
◆ Zero major safety accidents since 18 years
3、60 technicians of all kinds
60 technicians of all kinds
◆ We can design and build high level GMP workshop class A and class 1 clean room
◆ Tailor-made design solutions
◆ Project design are strictly implement GB50073-2013\GB 50473-2008\GB50457- 2008 and other national standard clean specification and the United States federal 209E standard


Third party acceptance test verification
4、Third party acceptance test verification
◆ Self-provided full set of testing instruments, first self-test after third-party testing
◆ The project construction strictly implements national GMP, US FDA and EU Euro GMP and other domestic international standards.
Customer Focused
5、Customer Focused
◆ Complete standardized process to ensure the construction period and quality
◆ One year after the warranty period, take the initiative to visit 2 times and 1 time at home
◆ We are convinced that our work is only valuable if the users are satisfied.




