Purification project
热门关键字:食品净化车间 医药洁净车间 低温车间 洁净室 无尘洁净室
Class 10,000 clean workshop decoration-Lexin
Category:
Project Description
Loxin Medical InstrumentsClass 10,000 clean workshopDecoration
Project Name:Zhejiang Loxin Medical Technology Co.
Project content:Class 10,000 clean workshop decoration
Industry Category:Medical Devices

■Introduction of Loxson
Ltd. was established on July 21, 2011, and its business scope includes the following licensed items: Class III: 6815 injection and puncture instruments, 6821 medical electronic instruments and equipment, 6825 medical high-frequency instruments and equipment, 6845 extracorporeal circulation and blood processing equipment, 6846 implantable materials and artificial organs, 6854 operating room, emergency room, treatment room equipment and apparatus, 6863 dentistry materials, 6864 medical hygiene materials and dressings, 6865 medical suture materials and adhesives, 6866 medical polymer materials and dressings, 6877 interventional equipment, etc.

Loxin's purification workshop meets the requirements of GMP certification, the cleanliness level of GMP workshop is 10,000 and 100,000, the area of purification workshop is 3800 square meters, the temperature is 18-26 degrees, the humidity is 55±10%, the purification workshop adopts air-cooled heat pump unit plus combined purification constant temperature and humidity air conditioning processing unit; supporting Siemens intelligent control system.



■ Key Notes:
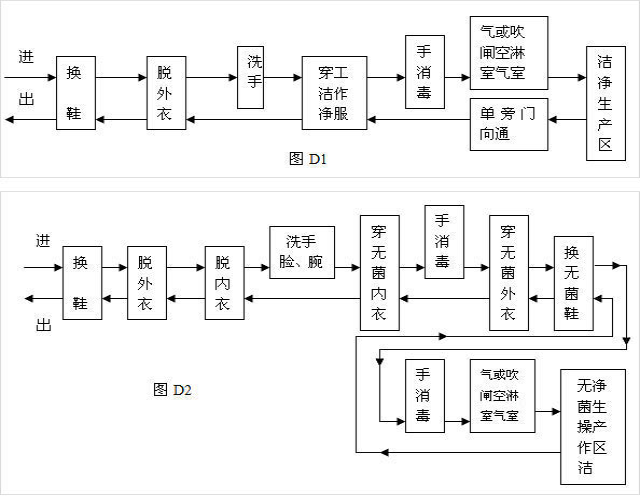
1、Personnel purification
General procedures for personnel entering and leaving the clean production area
The general procedures for personnel entering and leaving the clean production area and aseptically operating the clean production area are shown in Figures D1 and D2.
2、 Material purification (same as biomedical industry)
3, sterile medical device production environment cleanliness setting guidelines
3-1, in vivo implants, direct or indirect contact with circulating blood, bone cavity sterile instruments and components processing area should be at no less than 100,000 level. Sterile medical instruments and parts processing area implanted into the blood vessels should be at no less than 10,000 level (preferably with 100 level).
3-2, the above provisions outside the sterile medical instruments and parts processing area should not be less than 300,000 levels
3-3, direct contact with the use of the surface of the product, without cleaning that is used in the product primary packaging, the production environment and the product production environment clean level is the same (as far as possible in the same area).
3-4, the use of aseptic manipulation techniques for processing of sterile instruments implanted in the body should be produced in the local 100 level clean room under the 10,000 level.
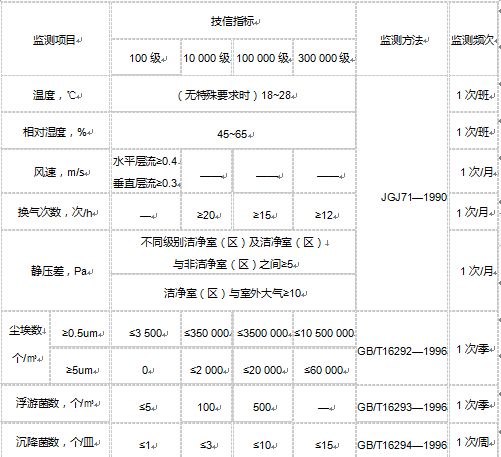
4, sterile medical apparatus clean room environmental requirements and monitoring
5, sterile medical apparatus clean room air cleanliness level table
| Cleanliness level | Maximum allowable number of dust particles, pcs/m³ | Maximum allowable number of microorganisms | ||
| ≥0.5 um | ≥5 um | Settling bacteria, pcs/dish | Planktonic bacteria,pcs/m3 | |
| Level 100 | 3500 | 0 | 1 | 5 |
| Level 10000 | 350000 | 2000 | 3 | 100 |
| 100000 level | 3500000 | 20000 | 10 | 500 |
| 300000 level | 10500000 | ≤60000 | 15 | -- |
Choose us Five advantages

Focus on food and drug, electronic industry clean room one-stop solution 18years since the establishment of a professional safety management system
18 years industry experience
1、18 years industry experience
◆ 18 years of precipitation
◆ 200 successful cases
◆ Repeat customers and word-of-mouth customers account for a high percentage
◆ Increasing number of well-known corporate clients


Construction Level 2 Qualified Enterprise
2、Construction Level 2 Qualified Enterprise
◆ Decoration Level 2 Qualification, Mechanical and Electrical Level 3 Qualification, General Contractor of Construction Level 3 Qualification
◆ Have a safety production license
◆ Self-built professional safety management system
◆ Zero major safety accidents since 18 years
3、60 technicians of all kinds
60 technicians of all kinds
◆ We can design and build high level GMP workshop class A and class 1 clean room
◆ Tailor-made design solutions
◆ Project design are strictly implement GB50073-2013\GB 50473-2008\GB50457- 2008 and other national standard clean specification and the United States federal 209E standard


Third party acceptance test verification
4、Third party acceptance test verification
◆ Self-provided full set of testing instruments, first self-test after third-party testing
◆ The project construction strictly implements national GMP, US FDA and EU Euro GMP and other domestic international standards.
Customer Focused
5、Customer Focused
◆ Complete standardized process to ensure the construction period and quality
◆ One year after the warranty period, take the initiative to visit 2 times and 1 time at home
◆ We are convinced that our work is only valuable if the users are satisfied.




